New work in optogenetics "creates a powerful tool that allows neuroscientists to apply a brake in any specific circuit with millisecond precision, beyond the power of any existing technology," said Thomas Insel, MD, director of the National Institute of Mental Health (NIMH).
The first paper on optogenetics, published in 2005, introduced channelrhodopsins: light-activated ion channels that can instantly activate neurons in which they are genetically expressed. The method has revolutionized research involving brain cells as well as heart cells, stem cells, and others that can be regulated by electrical signals. However, the ability to inactivate neurons with equal reliability and efficiency has been elusive. Now, a paper by Howard Hughes Medical Institute (HHMI) early career scientist Karl Deisseroth, M.D., Ph.D., and his team at Stanford University (CA) describes how modification of the ion channel allows neurons to be "switched off" with unprecedented control.1 "We're excited about this increased light sensitivity of inhibition in part because we think it will greatly enhance work in large-brained organisms like rats and primates," said Deisseroth, who serves as D. H. Chen Professor of Bioengineering and of Psychiatry and Behavioral Sciences at Stanford.
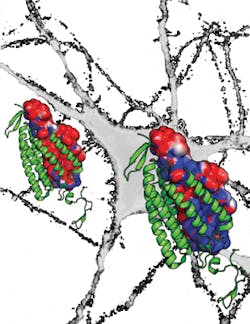
After years of searching for a naturally occurring light-sensitive channel with a pore permeable to negatively charged ions proved fruitless, the team embarked on a long-term project to modify the molecular structure of channelrhodopsin to allow negative ions into the cell. Not only does the new channel—named iC1C2 for "inhibitory C1C2" (C1C2 is a chimera of channelrhodopsin)—allow the selective passage of chloride ions, it greatly reduces the likelihood of action potentials by making the neuron more "leaky," a function not previously possible.
A final mutation to a cysteine residue in iC1C2 makes the channel both bi-stable and orders of magnitude more sensitive to light. When activated by blue light, the mutated channels remain open for up to minutes at a time, while exposing the channels to red light makes them close quickly. This level of long-term control is useful in developmental studies where events play out over minutes to hours. The long-channel open times also mean that neurons can essentially integrate chloride currents over longer time scales and, therefore, weaker light can be used to inhibit the neurons. Increased light sensitivity translates to less light-induced damage to neural tissue, the ability to reach deep brain structures, and the possibility of controlling brain functions that involve large regions of the brain.
"This is something we've sought for many years and it's really the culmination of many streams of work in the lab-crystal structure work, mutational work, behavioral work-all of which have come together here," Deisseroth says. Thomas Insel of NIMH, which funded the study, said the work will help researchers to better understand the brain circuits involved in behavior, thinking, and emotion. "This latest discovery by the Deisseroth team is the kind of neurotechnology envisioned by President Obama when he launched the BRAIN Initiative a year ago."
Merab Kokaia, Ph.D., of Lund University Hospital (Sweden), who has used optogenetics to study epilepsy and other conditions in rodent models, noted that the work could be not only very useful for behavioral studies in animals, but potentially, too, as an effective treatment alternative for neurological conditions where drugs do not work, such as some cases of severe epilepsy and other hyper-excitability disorders.
1. A. Berndt, S. Y. Lee, C. Ramakrishnan, and K. Deisseroth, Science, 344, 6182, 420–424 (2014); doi:10.1126/science.1252367.