James E. Evans, Paul Blackborow, Stephen F. Horne, and Jeff Gelb
While transmission electron microscopy can reveal structural details incredibly well, it only reliably reconstructs three-dimensional (3D) volumes of cellular material with a spatial resolution of 1–5 nm from samples less than 500 nm thick.1 Since most biological cells are 2–30 times thicker than this, a cell must be cut into consecutive slices with each slice reconstructed individually in order to approximate the contextual information of the entire cell.
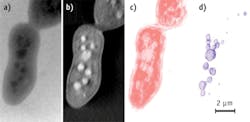
Soft x-ray tomography within the "water window" (that is, the wavelength range for which water is transparent to x-rays while other elements such as carbon and nitrogen absorb) has tremendous penetration power, and enables direct imaging of biological specimens up to 10 μm thick—20 times that of transmission electron microscopy. The technique can image intact cells and tissues on the micrometer or larger scale with tens to hundreds of nanometers spatial resolution.2 (See Fig. 1.) While soft x-ray microscopy has been extensively developed in synchrotron facilities, advancements in laboratory x-ray source designs now increase its accessibility by supporting commercial systems suitable for a standard laboratory.
Soft vs. hard x-rays
X-ray tomography can be performed using both hard and soft x-rays. While there is no fixed definition that distinguishes the two types, for this purpose we can say that hard x-rays have wavelengths shorter than 0.2 nm and soft x-rays have longer wavelengths.
Soft x-ray tomography can generate contrast—orders of magnitude greater than electron microscopy—natively on cellular material, enabling direct imaging of unstained cellular material without sectioning, freeze-drying, embedding, staining, or chemical fixation required by other electron approaches. The cellular contrast relates to the fact that carbon is detectable but oxygen appears transparent at x-ray energies greater than the k-absorption edge of carbon and less than the k-absorption edge of oxygen (see Fig. 2). For example, at 420 electron volts (eV)—which is well within the water window—carbon has a scattering cross-section 10x that of oxygen, while at hard x-ray energies, such as 10 keV, the ratio is closer to unity.3Both soft and hard x-ray tomography can be performed at either room temperature or cryogenic temperatures. The coupling of cryogenic sample preparation, transfer, and imaging, however, enables structural preservation of the sample of interest and allows for correlative imaging of the same cell using other instruments, including fluorescence and electron microscopes.4,5 The use of cryogenic temperatures also helps to preserve samples in a frozen hydrated state surrounded by amorphous non-crystalline ice,6 and to dynamically immobilize the samples to lock them into single conformations, which facilitates alignment of correlative datasets. Additionally, cryogenic sample preparation avoids potential deleterious structure artifacts caused by chemical fixation, freeze-drying, and polymer embedding, while also enabling higher x-ray doses for imaging that further improves contrast and resolution.
Increased access with bench-top sources
In recent years, soft x-ray computed tomography using synchrotron radiation light sources has enabled visualization of sub-cellular structures at up to 36 nm spatial resolution.7-9 Unfortunately, access to synchrotron light sources for whole cell tomography is limited, as is the opportunity to perform correlative work or optimize a variety of sample preparation conditions. While synchrotron-based transmission x-ray microscopes have fostered the development of whole cell x-ray tomography, the widespread adoption of the technique will benefit from less expensive and more readily accessible x-ray microscopes that incorporate compact light sources suitable for home institutions.
Recently, two cryogenic soft x-ray microscopes using compact light sources have been reported in the literature. The first makes use of a custom liquid-jet high-brightness laser-plasma source to acquire images in less than 10 seconds,10 while the second uses a commercially available Z-pinch light source capable of single image acquisition in 30 seconds.11 Both of these systems were able to reconstruct similar sub-cellular structures around 100 nm in diameter through cellular thicknesses of up to 5 μm.
By comparison, cryogenic soft x-ray tomography using third-generation light sources can acquire a single image in just one second and image through thicknesses up to 10 μm.7 It should be noted that current bench-top sources do not have the same brightness or coherence as third-generation synchrotrons, and thus the improved access of compact sources comes with a trade-off of exposure time. However, there are both soft and hard x-ray compact sources that currently rival the brightness of second-generation synchrotrons, and the technology is expected to advance even further in coming years with the incorporation of brighter or more coherent compact sources.12
Soft x-ray light in a cryogenic microscope
The second cryogenic soft x-ray microscope platform mentioned above is commercially available (Xradia Inc.; Pleasanton, CA) and includes cryogenic transfer and imaging capabilities (see Fig. 3). It can function either as a stand-alone lab instrument in combination with a laboratory x-ray light source, or as a synchrotron beam-line end station. The laboratory-based UltraXRM-L220c microscopy system, which measures approximately 15 × 6 × 4 ft., uses a compact light source based on a patented electrodeless Z-pinch design (Energetiq Technology Inc.; Woburn, MA).Traditional Z-pinch plasma sources use electrodes to conduct high-voltage (~1–2 kV), high-current (~10 kA) pulses into the plasma. This high electrical and thermal load, combined with ion sputtering from the plasma, causes the electrodes to be rapidly eroded, thereby coating any nearby optical components with debris that rapidly degrades optical performance. Electrodes need to be replaced frequently, driving up operating cost and lowering reliability. Laser plasma sources, where a jet of liquid nitrogen is heated with pulses from a high-performance laser, can offer a cleaner source of photons. However, achieving high spatial stability for the jet has proved challenging, and often these sources demonstrate reliable operation only for few hours.
The electrodeless design avoids the electrode erosion problem of the traditional Z-pinch sources by inductively coupling the high-voltage/high-current pulses into the plasma. The spatial stability challenge seen in laser plasma sources is not observed, as the inductively coupled plasma magnetically pins the plasma to within a few micrometers. The electrodeless Z-pinch sources operate for 20 days of 24/7 operation between maintenance, a process that involves the simple replacement of the liner (or "bore") of the Z-pinch containing region. The source uses nitrogen as the plasma gas, generating soft x-rays at photon energies of 430.75 eV (corresponding wavelength of 2.8787 nm).13 This photon energy is within the water window, so hydrated and frozen-hydrated carbon-based materials can be imaged with high contrast due to different absorption between carbon-based cellular material and the surrounding oxygen-rich water/vitreous ice.14
The photons are initially focused onto the specimen by a reflective condenser lens, after which the scattered light is refocused by a diffractive objective lens (Fresnel zone plate) to form an image with resolution better than 50 nm (see Fig. 4). Vitrified samples enter the imaging chamber via an integrated loading station that maintains the sample at 110 K to ensure that cryogenic conditions are continually satisfied during transfer and imaging. The sample stage accommodates such standard sample-support geometries as flat, 3.0 mm grids or pin mounts, creating an optimizable platform for interrogating organic and biological samples in near-native environments.Biological benefit
Since laboratory-based cryogenic soft x-ray microscopes permit imaging whole cells at intermediate-length scales, they help bridge the resolution gap between light and electron microscopy—two widely used techniques for molecular and structural biology research. Therefore, several types of samples can immediately benefit from an expansion of x-ray tomography capabilities, including research with single microorganisms, microbial communities and biofilms, viruses, and enucleated or serum-starved eukaryotic cells. For example, the field of view of compact soft x-ray tomography is perfectly suited for studying microbial biofilm architecture as a function of depth from the natural substrate interfaces or probing changes in the structure of components responsible for photosynthesis and nitrogen fixation in cyanobacteria. Additionally, the wide field of view for compact soft x-ray tomography could provide greater sampling and better statistics for research devoted to linking genetic mutations with structural changes in large purified organelles of heterogeneous morphology or size, such as isolated mitochondria or chloroplasts.
Finally, cell lines used for pancreatic, prostate, and breast cancer research can be serum-starved or enucleated to promote cellular flattening. This permits analysis by compact soft x-ray tomography to identify morphological variations of sub-cellular components or changes in internal localization that exist between healthy, cancerous, or pharmaceutically treated cells. Similar experiments on single cells or cell monolayers can be performed to better understand the health effects associated with exposure to a wide range of nanomaterials. Moreover, cancer-based and nanotoxicology research can even be extended to tissue biopsies following cryo-ultramicrotomy of high-pressure frozen samples15 that yield serial sections suitable for imaging.
While these experiments represent just a fraction of the types compatible with a compact cryogenic soft x-ray microscope, they illustrate the versatility of the approach and the potential impact that could be realized from extensive implementation.
ACKNOWLEDGEMENTS
Funding support was provided by NIH grant number 5RC1GM091755 to JEE and 2R44RR022488 to Energetiq Technology, Inc. and SFH. A portion of this work was performed at the Pacific Northwest National Laboratory, which is operated by Battelle Memorial Institute for the U.S. Department of Energy under Contract No. DE-AC05-76RL01830.
REFERENCES
1. J. L. Milne and S. Subramaniam, Nat. Rev. Microbiol., 7, 666–675 (2009).
2. J. Kirz, C. Jacobsen, and M. Howells, Q. Rev. Biophys., 28, 33–130 (1995).
3. C. T. Chantler, J. Phys. Chem. Ref. Data, 24, 71–591 (1995).
4. G. Schneider, P. Guttmann, S. Rehbein, S. Werner, and R. Follath, J. Struct. Biol., 177, 212–223 (2012).
5. D. B. Carlson and J. E. Evans, J. Vis. Exp., 52, 2909 (2011).
6. M. Adrian, J. Dubochet, J. Lepault, and A. W. McDowall, Nature, 308, 32–36 (1984).
7. C. A. Larabell and M. A. Le Gros, Mol. Biol. Cell, 15, 957–962 (2004).
8. D. Y. Parkinson, G. McDermott, L. D. Etkin, M. A. Le Gros, and C. A. Larabell, J. Struct. Biol., 162, 380–386 (2008).
9. G. Schneider et al., Nat. Meth., 7, 985–987 (2010).
10. H. M. Hertz et al., J. Struct. Biol., 177, 267–272 (2012).
11. D. B. Carlson, J. Gelb, V. Palshin, and J. E. Evans, Microsc. Microanal., 19, 1, 22–29 (2013).
12. S. Kneip et al., Nat. Phys., 6, 980–983 (2010).
13. S. F. Horne, J. Silterra, and W. Holber, J. Phys. Conf. Ser., 186 (2009).
14. C. A. Larabell and K. A. Nugent, Curr. Opin. Struct. Biol., 20, 623–631 (2010).
15. A. Al-Amoudi, L. P. Norlen, and J. Dubochet, J. Struct. Biol., 148, 131–135 (2004).
James E. Evans is Staff Scientist with the Pacific Northwest National Laboratory, Environmental Molecular Sciences Laboratory, Richland, WA (www.emsl.pnl.gov); Paul Blackborow is CEO and Stephen F. Horne is Senior Scientist at Energetiq Technology, Inc., Woburn, MA (www.energetiq.com); and Jeff Gelb is Senior Applications Development Engineer at Xradia Inc., Pleasanton, CA. Contact Evans at [email protected] or Blackborow at [email protected].
![FIGURE 2. This comparison of atomic scattering cross-sections shows x-ray photoelectric, elastic, and Compton plots for carbon as a function of wavelength. Photoelectric absorption provides high contrast for organic material in the soft x-ray regime (including the 'water window'), while Compton scattering of photons by free electrons becomes dominant for hard x-rays [2]. FIGURE 2. This comparison of atomic scattering cross-sections shows x-ray photoelectric, elastic, and Compton plots for carbon as a function of wavelength. Photoelectric absorption provides high contrast for organic material in the soft x-ray regime (including the 'water window'), while Compton scattering of photons by free electrons becomes dominant for hard x-rays [2].](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2015/12/1303bowevansf2.png?auto=format,compress&fit=max&q=45&h=241&height=241&w=250&width=250)

