Yang Pu, Samuel Achilefu, and Robert R. Alfano
The key advantages of optical imaging compared with other modalities are superior sensitivity, extremely low energy radiation, the ability to monitor multiple independent optical biomarker reporters simultaneously, and relatively simple imaging hardware.1 Optical techniques provide an accurate and rapid means to detect regions of cancer,2 but a challenge in optical cancer screening is to locate tumors lying deep within the body's organs. The spectral ranges of absorption and emission of most native fluorophores (tryptophan, collagen, elastin, NADH, flavin, etc.) within tissue run from ultraviolet (UV) to visible. A spectral window in the far-red to near-infrared (NIR) range (650–1100 nm) corresponds to low absorption and scattering of light by tissue and allows light to penetrate up to several centimeters into tissue.
Biomarkers/receptors that are over-expressed in cancerous tissues but down-regulated in healthy tissues enable imaging and selective surgery. The primary goals are detection of cancer, complete removal of tumors, and accurate delineation of margins between normal and cancerous tissues.
Optical 'smart beacons'
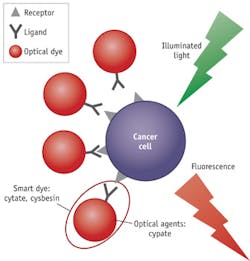
By conjugating to a probing ligand,3 optical beacons such as fluorescent dyes,4 metallic nanoparticles (NPs),5 and semiconductor quantum dots (QDs)6 become "smart" and can be used to target and identify specific, over-expressed, or up-regulated elements in carcinogenesis, or the markers associated with underlying biological processes such as metastasis. The ligands, including antibodies, aptamers, and peptides, can be used as delivery vehicles to enhance tumor detection with high specificity. They locate the over-expressed receptors on the cancer cells, and then the smart agent docks on the cells and reports (by way of fluorescent glow) the cancer location (see Fig. 1).
This approach has several advantages vs. nonspecific contrast agents, including the enhancement of localization in tumors, rapid clearance from healthy tissue, the possibility of preparing a library of peptides for rapid identification of bioactive molecules, and preservation of the spectral advantages of the reporter dyes. We will look at two kinds of smart beacons based on indocyanine green (ICG)—cytate (cypate-octreote peptide analog conjugate) and cybesin (cypate-bombesin peptide analog conjugate)—and explore key ideas behind their application to enhance optical imaging for cancer detection and tumor resection guidance.
The birth of cypate
The U.S. Food and Drug Administration (FDA)-approved ICG (see Fig. 2a) is the most commonly used dye in the NIR spectral "tissue optical window,"1 and its contrast enhancement has been reported for detection of mammary tumors in both animal models and in humans clinically. ICG is not designed for specific targeting of cancer cells, however; in fact, studies in animal models conclude that there is no specific mechanism of action for this dye to localize in tumors.3
Researchers at Washington University School of Medicine developed and synthesized the dye cypate, whose synthesis was also reported elsewhere.2,3 Compared with ICG, the main advantage of cypate is the carboxylic group in its molecular structure, which makes it reactive (see Fig. 2b). Usually, an antibody or peptide has an amino group, which can be conjugated with the carboxylic group of cypate to form an amide bond through simple chemical reaction. This property makes cypate easily engineered into a smart beacon by preparing a library of ligands (peptides or antibodies) for rapid identification of bioactive molecules. The conjugation of a ligand to cypate produces a smart beacon that can target specific receptors over-expressed in cancerous cells.The selection of the ligands is based on the expression conditions of receptors in the different types of cancer cells. For example, somatostatin receptors (SSTR) are known for predominant expression in several human adenomas such as somatotroph and lactotroph, and neuroendocrine tumors including human prostate cancer.7 They can be used as a basis for in vivo tumor targeting. Bombesin belongs to a family of brain-gut peptides that play an important role in cancer development: Human primary tumors can synthesize bombesin, and the bombesin receptor can be over-expressed on the membranes of human prostate cancer cells.4
Cypate conjugation for targeting
Conjugating cypate with octreotide produces cytate (whose ligand is an octreotide peptide analog), while conjugation with a bombesin peptide analog generates cybesin (whose ligand is a bombesin peptide analog). These new synthetic smart contrast agents have been validated to target SSTR and bombesin receptors in animal model3 and human tissues,4 respectively. The basis of cytate and cybesin (see Figs. 2c and 2d) as smart contrast agents for human prostate cancer depends on two factors: First, the high affinity of octreotide or bombesin peptide for the somatostatin and bombesin receptors, and second, the over-expression of these receptors in human prostate cancer cells.7 Specific ligands targeting the corresponding cancer over-expressed receptors will be identified with the desired fluorescence dye; therefore, the synthesized specific cancer cell-targeted contrast agents (optical reporter + ligand) can function as smart dyes to light up the tumor. Previous studies indicated that the cytate can be used as a smart beacon to target prostate5 and breast8 cancer in human tissues, and kidney cancer in small animal model.2 Cybesin has been validated to target prostate cancer in human tissues4 and pancreatic cancer in small animal model.2
Testing the preservation of absorption and fluorescence spectra for cybesin and cytate revealed that both agents possess the spectral advantages of ICG (see Figs. 3a and b): The absorption band of both are from 680 nm to 830 nm with a strong peak at 792 nm for cybesin and 789 nm for cytate. The fluorescence spectra cover from 800 nm to 950 nm with a main peak at 825 nm for cybesin and 837 nm for cytate. These ranges are in the NIR range of the "tissue optical window."Optical imaging techniques
Optical smart beacons can identify morphological and functional alterations due to cancer growth, but they require the help of imaging instrumentation to noninvasively access the optical fingerprint reported by the specific dye with the desirable color (wavelength). Optical imaging systems are able to visualize specimens from the cellular and subcellular levels (using microscopy) to entire bodies in small animals and organs in humans using macroscopic planar (2D) and tomographic (3D) techniques. Optical spectral polarization imaging is a common and simple technique for capturing fluorescence images using a charge-coupled device (CCD) camera and lasers or white light with bandpass filters to excite smart dyes.
In a test of optical spectral imaging to evaluate the efficacy of Cytate (cypate-octreotate conjugate) in male Lewis rats bearing subcutaneously implanted SSTR positive CA20948 pancreatic tumor xenograft, results showed remarkable retention of Cytate in CA20948 tumors (see Fig. 5).2Cytate has potential to receive regulatory approval for use in humans because the analogous peptide (octreotide) and optical reporter (ICG-derivative) are FDA-approved. Most recently, the over-expression of folic acid receptors on the epithelium of ovarian cancers is used with fluorescein dye attached to locate and remove cancers during surgery.9
Further progress
The examples of receptor-targeting contrast agents highlighted here represent the outcome of more than two decades of collaboration between the Institute for Ultrafast Spectroscopy and Lasers at the City College of New York and Optical Radiology Lab at Washington University. Focusing on the intersection of multiple independent research fields has enabled development of novel optical instruments and tools of vastly improved efficiency, and their application to meet current challenges in early cancer detection or differentiation between aggressive cancer and indolent disease. Research continues to progress towards translating tumor-specific molecular beacons from benchtop to bedside through development of novel smart contrast agents, advanced image reconstruction algorithms, and high-end time-resolved optical tomography systems.10
ACKNOWLEDGEMENTS
This research is supported by U. S. Army Medical Research and Material Command grants numbered W81XWH-11-1-0335, W81XWH-08-1-0717, and DAMD17-01-1-0084 and National Institutes of Health NIBIB R01 EB008111 and NCI R01 CA171651.
REFERENCES
1. Y. Pu et al., Technol. Cancer Res. Treat., 10, 6, 507–517 (2011).
2. S. Achilefu, Technol. Cancer Res. Treat., 3, 393–409 (2004).
3. S. Achilefu, R. B. Dorshow, J. E. Bugaj, and R. Rajagopalan, Invest. Radiol., 35, 479–485 (2000).
4. Y. Pu, W. B. Wang, S. Achilefu, and R. R. Alfano, Appl. Opt., 50, 7, 1312–1322 (2011).
5. J. Xue et al., Biosens. Bioelectron., 41, 71–77 (2013).
6. X. Gao and S. Nie, Trends Biotechnol., 21, 371–373 (2003).
7. Y. Pu, W. B. Wang, B. B. Das, S. Achilefu, and R. R. Alfano, Appl. Opt., 47, 2281–2289 (2008).
8. L. A. Sordillo et al., "Time resolved fluorescence for breast cancer detection using an octreotate indocyanine green derivative dye conjugate," Proc. SPIE, 8577, Optical Biopsy XI, 857708 (Mar. 19, 2013); doi:10.1117/12.2003102.
9. G. M. van Dam et al., Nat. Med., 17, 1315–1319 (2011).
10. M. Niedre et al., Proc. Nat. Acad. Sci. U.S.A., 105, 19126–19131 (2008).
Yang Pu is a post-doctoral scholar at the Institute for Ultrafast Spectroscopy and Lasers (IUSL) and the departments of electrical engineering and physics at City College of the City University of New York (CUNY; New York, NY); www1.ccny.cuny.edu/ci/iusl. Samuel Achilefu is director of the Optical Radiology Laboratory and a professor in the Department of Radiology at the Washington University School of Medicine (St. Louis, MO); http://orl.wustl.edu. Robert R. Alfano is director of CUNY's IUSL and Distinguished Professor in the department of physics. Contact Prof. Alfano at [email protected].