ByKengyeh K. Chu, Susan E. Birket, Linbo Liu, Steven M. Rowe, and Guillermo J. Tearney
With a resolution of 1–2 μm, μOCT is the only noninvasive method able to comprehensively and simultaneously study both the subcellular structures and functions important for diseases such as cystic fibrosis (CF), and the correlations between them, in vivo. Thus, μOCT promises to become an important new tool for understanding the mechanisms of such pathologies.
A new, high-resolution form of optical coherence tomography (OCT), micro-OCT (μOCT) images at 1–2 μm—sufficient to visualize individual cells and even subcellular features in vivo. Developed by the Tearney Lab at the Massachusetts General Hospital in collaboration with Dr. Steven Rowe at the University of Alabama at Birmingham, μOCT was originally demonstrated for imaging atherosclerosis, where microscopic features (e.g., cholesterol crystals, macrophages, platelets, and fibrin) were revealed in arterial plaques.1 More recently, the technique is being applied to investigate airway surfaces, where tiny, hair-like organelles known as cilia protrude approximately 7 μm and beat in a whipping motion 10 to 20 times per second to carry dirt- and bacteria-trapping mucus away from the lungs.2 This process, known as mucociliary clearance, is sometimes impaired by disease, which leads to increased incidence of infection, airway obstruction, and decreased lung function.
Cystic fibrosis (CF) is perhaps the best-known example of a disease associated with mucus transport impairment.3 Affecting 30,000 patients in the United States and 70,000 globally, CF results from mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which acts primarily as a chloride and bicarbonate ion channel. The cell membranes of CF patients are abnormally impermeable to these anions. CF lungs are characterized by impaired mucociliary transport, highly viscous mucus, and are prone to frequent and persistent infection. Even though the genetic defect and the resulting pulmonary consequences are understood, the intermediate links in the chain—including the mechanism that causes mucus transport to break down—remain unknown.
Imaging cilia motion
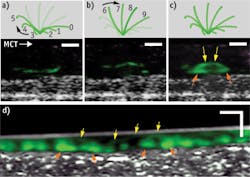
While living cilia have been difficult to study noninvasively because of their small size and high rate of motion, μOCT offers the resolution and speed to image them directly. The tip of a single cilium can be imaged as it progresses through its whip-like motion, which can be divided into effective and recovery strokes. Figure 1 demonstrates this: Panel A shows the recovery stroke of the cilium; on μOCT, the cilium tip, colored in green, traces a path close to the cell surface as it arcs backwards in preparation for the effective stroke, shown in Panel B. In this stroke, the cilium tip travels forward at a greater height than during the recovery stroke. Panel C illustrates the full cycle of the cilium through one full period, as the complete arc trajectory of the tip is seen. Panel D shows a time-averaged region of densely ciliated swine epithelium in which the stroke arc extents are also observed.
For CF research, several quantitative measures are often used to characterize the functional health of the respiratory epithelium and consequential mucus clearance. Two such metrics are airway surface liquid (ASL) and periciliary liquid (PCL) depth, which together indicate the hydration status of the airway surface. The ASL depth quantifies the total thickness of mucus overlying the cells, and the PCL depth measures the thin sheet of liquid gel that encompasses the cilia; it is primarily within the PCL that the cilia beat. The ciliary beat frequency (CBF) is a measure of the cilia stroke rate. The mucociliary transport (MCT) velocity quantifies the objective of mucociliary clearance: the removal of mucus. MCT rate is the speed at which mucus is conveyed over airway surface.
These four parameters (ASL depth, PCL depth, CBF, and MCT velocity) are essential metrics for analyzing CF because diseased airways often exhibit dehydration (depressed ASL and PCL), lagging ciliary beat (reduced CBF), and of course mucus clearance difficulty (significantly decreased MCT). Though methods are available to measure each of these parameters in cultured cells or tissue, no pre-existing technique is able to quantify all of these metrics together.
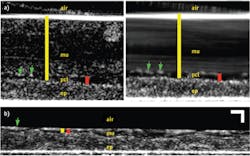
Fortunately, in addition to the already described imaging of individual cilia, μOCT is able to quantify each of these measurements, as demonstrated in Fig. 2, which shows μOCT images of cultured ciliated airway cells derived from a healthy patient.4 Because μOCT is a cross-sectional technique, the thicknesses of the ASL and PCL can be easily measured from images. The yellow bar in Fig. 2a is drawn between the bright line that represents the top of the mucus layer and the top of the epithelium, and corresponds to the thickness of the ASL layer. The red bar similarly indicates the PCL layer, which can be more distinctly seen on a time-averaged image (right side). As μOCT can image the up-and-down periodic motion of cilia tips (green arrows in Fig. 2a), the frequency of the beat (CBF) can also be directly determined from μOCT movies. Finally, as particles in the mucus are swept across the μOCT field of view by mucociliary clearance, the speed of their motion defines the rate of mucociliary transport (MCT). The streaking pattern within the mucus on the time-averaged right-hand image of Fig. 2a results from the motion of the particles seen within the mucus on the left-hand image.
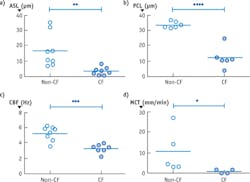
Figure 2b depicts an image from cells taken from a CF patient and cultured. This μOCT data shows very different characteristics compared to the healthy sample. The mucus layer is very thin and the cilia-containing PCL layer is compressed as well. The cilia also beat more slowly and mucus transport is significantly reduced in the CF case. Figure 3 shows the quantitative differences observed in the non-CF and CF cells.
Revealing relationships
Thus, μOCT is the only method available to comprehensively and simultaneously study airway hydration, ciliary function, and mucus transport, and μOCT's ability to derive measurements from the same regions of the same samples at the same time makes it possible to study correlations between these factors. Because the cause-and-effect relationship between parameters (e.g., airway hydration and transport rate) can be determined only by studying them together, μOCT has the potential to be an important new tool for understanding the mechanisms of CF and other ciliary disorders.
In a recent study, μOCT was used to analyze the relationship between the periciliary liquid (PCL) and the rate of mucus transport (MCT), which is possible only using a method that can capture both measurements at once.4 In normal airways, it is expected that a higher amount of liquid in the PCL is associated with higher MCT rates, reflecting the importance of hydration for mucus transport. And indeed, μOCT imaging revealed a positive correlation between PCL thickness and MCT rate in normal explanted airways.4 However, μOCT also showed that this relationship was disrupted and even reversed in CF samples, indicating that a factor beyond airway hydration alone is responsible for delaying mucus transport in CF, and the slight negative slope in the correlation even indicates that more PCL hydration is associated with decreased mucus transport.4 These results suggest that there is an innate defect in the CF mucus itself such that more of its presence is, in fact, counterproductive.
One possible explanation for this is that CF mucus is inherently more viscous. Though elevated viscosity of expectorated CF sputum is a well-established fact, it is not known whether the viscosity increase is inherent or results from other CF-related phenomena such as chronic infection or mucociliary clearance failure. One hypothesis suggests that a mucus viscosity anomaly in CF can be caused by a decreased concentration of bicarbonate ions (also resulting from CFTR dysfunction), disrupting the ability of mucins (the long molecular chains that mesh around a watery medium to form mucus) to unfold and causing them instead to stick together to form an abnormally viscous mucus.5 Using μOCT to test this hypothesis, we analyzed the response of the PCL/MCT relationship following application of the bicarbonate transport inhibitor 4,4'- dinitrostilbene-2,2'-disulfonic acid (DNDS) to normal airways. Indeed, μOCT measurements showed that the PCL/MCT relationship is inverted when bicarbonate is inhibited, duplicating the CF observation. The viscosity of mucus was also found to be elevated in both CF and bicarbonate-inhibited airways, which suggests that bicarbonate inhibition does cause a viscosity increase in situ that could explain the newly discovered PCL/MCT inversion (see Fig. 4).
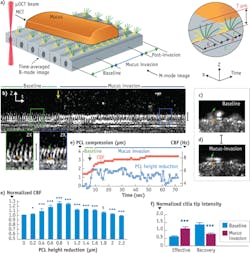
Another revelation enabled by μOCT was a previously unobserved phenomenon that occurs when cilia on normal cultured human airways cells are subjected to an increased workload when encountering mucus rafts.6 Imaging with μOCT showed that a small compression (up to 2 μm) of the cilia caused by a mucus load stimulates the cilia to beat faster and to transport mucus more rapidly (see Fig. 5). Further load that compresses the cilia beyond 2 μm negates this increase, and the beat frequency further declines in response to additional compression. Simultaneous fluorescence imaging revealed a calcium spike at the onset of the mucus load, and the application of BAPTA-AM (a chemical that binds calcium and thus reduces its availability to the cell) prevents the ciliary beat increase. These results indicate that a calcium-signaling-based feedback mechanism helps airways to cope with periods of increased load by increasing ciliary beating and that this feedback mechanism fails when the load is too great.
Recent and future discoveries
The ability of μOCT to measure multiple facets of airway function and mucus transport has proven to be very useful, and has already generated new discoveries enabled by simultaneous and co-localized measurements of mucus volume, ciliary beating, and transport rate. Furthering scientific understanding of the basic mechanisms of mucus transport, including characterizing the effects of diseases such as CF, clarifies suitable therapeutic targets for investigation. For example, the finding that the absence of bicarbonate transport is associated with failure of mucus clearance, despite adequate airway hydration in CF, suggests a pathway to address the viscosity or adherence of CF mucus itself.
Furthermore, μOCT could help identify new drugs by detecting beneficial changes to cultured airway cell systems induced by investigational substances, and quantitatively characterize the functional therapeutic effect of drugs that have shown promise in the laboratory or in human trials. μOCT can also characterize the airway function of new CF animal models to determine their suitability for representing human CF airway disease features, as recently done for the new CF rat.7
And now, μOCT endoscopic probes are being developed to characterize the same benchmarks of airway function in living patients. In vivo imaging will allow direct characterization of the progression of CF lung disease, mitigating the need for cell culture and animal models.
In addition, μOCT has potential as a clinical tool for diagnosing the severity of lung disease and monitoring responses to treatment. Besides CF, other common respiratory diseases that affect the airway epithelium or the mucociliary transport apparatus—for instance, primary ciliary dyskinesia (PCD) or chronic obstructive pulmonary disease (COPD)—can be investigated by μOCT imaging.
Acknowledgement
Figures 2, 3, and 4 are reprinted and Figure 5 is adapted with permission from the American Thoracic Society. Originally published in references 4 and 6. Copyright © 2014 American Thoracic Society.
References
1. L. Liu et al., Nat Med., 17, 1010–1014 (2011).
2. L. Liu et al., PLoS One, 8, e54473 (2013).
3. S. M. Rowe, S. Miller, and E. J. Sorscher, N. Engl. J. Med., 352, 1992–2001 (2005).
4. S. E. Birket et al., Am. J. Respir. Crit. Care. Med., 190, 421–432 (2014).
5. P. M. Quinton, Am. J. Physiol. Cell Physiol., 299, C1222-33 (2010).
6. L. Liu et al., "An autoregulatory mechanism governing mucociliary transport is sensitive to mucus load," Am. J. Respir. Cell Mol. Biol., in press (2014).
7. K. Tuggle et al., PLoS One, 9, e91253 (2014).
KENGYEH KEN CHU, Ph.D., is Research Fellow in Dermatology at Massachusetts General Hospital's Wellman Center for Photomedicine. SUSAN E. BIRKET, PharmD, Ph.D., is a postdoctoral trainee in Pulmonary, Allergy, and Critical Care Medicine at the University of Alabama at Birmingham (UAB). LINBO LIU, Ph.D., is Assistant Professor of Electrical and Electronic Engineering at the Nanyang Technical University in Singapore. STEVEN M. ROWE, MD, MSPH, is Associate Professor of Medicine, Pediatric Pulmonary Medicine, and Physiology & Biophysics at UAB and the Director of the UAB Cystic Fibrosis Transition Clinic. GUILLERMO J. TEARNEY, MD, Ph.D., is Mike and Sue Hazard Family MGH Research Scholar and Professor of Pathology at Harvard Medical School and the Wellman Center for Photomedicine. Contact Dr. Tearney at [email protected]; www.tearneylab.org.