DARPAN SHIDID, MARTIN LEARY, and MILAN BRANDT
Additive manufacturing is rising in importance globally because parts can be built directly from computer models or from re-engineered components, bypassing traditional manufacturing processes. Benefits include new designs not possible using conventional subtractive technology; dramatic savings in time, materials, energy, and other costs in producing new components; significant reductions in environmental impact; and faster time to market. Additive manufacturing refers to methods that generate three-dimensional structures layer by layer.1
The powder-bed process, which is known by the terms selective laser melting (SLM), direct-metal laser sintering (DMLS), and laser powder-bed fusion (LPBF), is regarded as an essential element of additive manufacturing, complementing other metal techniques. The process builds finished components from raw metal powder layer by layer through laser melting; the metal powder particles typically range from 25 to 50 µm in diameter. The metal powder is spread across a metal platform with typical dimensions of 280 × 280 mm with layer thickness being on the order of 30 to 50 µm. The platform is contained inside a chamber, which is flooded with argon gas during operation to minimize any oxide formation in the laser-generated melt pool. Once the powder is spread across the platform, a laser beam is scanned across its surface based on the component design and where the laser hits the powder surface, it melts it and fuses it to the layer below. Once the laser scan is completed, the platform descends by the powder layer thickness and the whole process is repeated until the part is manufactured. Functional or actual components can be built in their final material, minimizing material wastage, and samples and small product runs can be manufactured quickly at comparatively low cost.
Additive manufacturing processes are not subject to the constraints associated with traditional manufacturing methods and provide significant opportunities for the design of novel geometries and complex structures such as cellular structures. The design freedom offered by additive manufacturing may be used to enhance the strength-to-weight ratio of structural components by transforming solid geometry into a cellular structure or space-filling hollow sections of the model with a cellular structure. The cellular structure within the model may be useful in distributing the loads evenly compared to a hollow model while conforming to the geometric boundaries of the object.
This article discusses some of the basic concepts behind the metallic lattice structures manufactured using SLM technology and their application to the next generation of biomedical implants in particular at the RMIT Centre for Additive Manufacturing (Melbourne, Australia).
Cellular structures
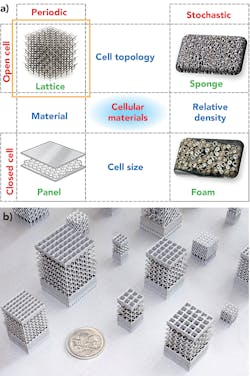
Cellular structures are categorized as aperiodic (or stochastic) and periodic. Aperiodic cellular structures are characterized by random orientations of structural elements and can be found in nature in abundance, such as observed in bones, sponges, bird beaks, and animal horns. Such cellular structures are often surrounded by a thick plate structure, such as cortex bone, and therefore are referred to as sandwich structures. FIGURE 1 shows examples of cellular structures.
Periodic structures, on the other hand, consist of a plurality of a single unit cell arranged in a specific pattern such as honeycomb structures and engineered lattice structures. A lattice structure, from a structural engineering context, is an array of struts that may be pin-jointed or rigidly bonded at their intersection2 and have been shown to have up to 300% greater strength compared to aperiodic cellular structures under similar circumstances. The generation of periodic lattice structures is computationally efficient, as it involves the repetition of predefined unit cell configurations.3 Furthermore, local and global properties of the lattice structure can be tailored by using applicable unit cells from a large unit cell library. This enables greater user-control over lattice configurations and density, making periodic lattice structures ideal for functionally graded materials. Additionally, the structural optimization of periodic lattice structures is computationally efficient compared to aperiodic structures.
Structural performance of lattice structures
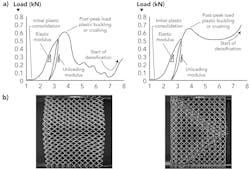
The failure of a lattice structure is a complex phenomenon that can be attributed to several associated failure modes and loading conditions, including yield and buckling failure modes. Structurally, lattice structures can be broadly classified into bending-dominated and stretch-dominated structures. FIGURE 2 shows typical compressive load-extension curves of stretch-dominated and bending-dominated structures. In both cases, the lattice structure undergoes plastic consolidation initially after which the load starts to increase rapidly. In stretch-dominated structures at peak load, the struts start to yield plastically and failure occurs either by buckling or fracture,4 resulting in a steep drop in load-carrying ability.However, in bending-dominated structures, the collapse of the structure occurs at nearly constant stress, making bending-dominated structures more suitable for energy-absorbing applications and for the emulation of trabecular bone, whereas stretch-dominated structures are desirable for high load-carrying applications. Lattice densification occurs after the structure is fully crushed and the structure behaves as a solid material.
Design and manufacture of lattice structures
Generating lattice structures for regular geometries, including orthogonal, prismatic, or symmetric objects, is relatively easy due to the object’s predictable geometry. However, irregular free-form geometries such as orthopedic implants are challenging to tessellate with periodic lattices, typically requiring the designer to trade-off structural integrity with associated lattice geometric conformity.
Additionally, additive manufacturing technologies make use of support structures to increase the scope of manufacturable geometries. In SLM, support structures are used as structural supports and provide a thermal path for conducting heat of fusion from the melt pool, thereby increasing cooling of the part and managing residual stresses.
Although support structures are useful for increasing additive manufacturability, they introduce challenges for part quality and cost. For example, in SLM, support structures are fused to the build part and are either frangible or removed by mechanical grinding, posing the risk of physical damage or deformation of the manufactured object. Secondly, support structures used within internal or hollow features (including lattice structures) cannot be removed with nondestructive methods, and thereby introduce further design constraints to additive manufacturing. Therefore, overhang geometries are considered undesirable due to the requirement of support structures and should be avoided.
However, elimination of overhangs is not feasible, especially in complex structures such as lattice structures and may result in structural instability if critical structural elements are eliminated. Therefore, designing geometries that are manufacturable without the use of support structures either results in extended modeling time or structurally unstable components. So, it is complex to determine the internal structural configuration for space-filling conformal lattice structures that are manufacturable using SLM as well as being structurally appropriate for the intended application. Research as RMIT University has shown that support-free manufacture of lattice structures is feasible with identification and accommodation of manufacturing constraints of the applied SLM technology.5
Traditional orthopedic implants
Traditionally, orthopedic implants have been mass-manufactured with a limited range in sizes and shapes. Additionally, regulatory strategy for device approval based on ‘substantial equivalence’ to predicate devices has hindered further structural and functional evolution of orthopedic implants. Key limitations associated with traditional orthopedic implants include:
- Mass-manufactured off-the-shelf orthopedic implants are made in specific size and shape groups and are not designed according to individual patients’ anatomy and biomechanics. Therefore, a patient’s bone is usually modified to fit the implant, which is contradictory to what is desirable.
- Load-bearing orthopedic implants have solid construction and are generally over-stiff compared to the surrounding bone.
- This stiffness mismatch results in stress-shielding, which causes resorption of the bone and subsequent failure.
- Standard implant designs require extensive implantation site preparation, resulting in excessive removal of healthy soft tissue and bone in some cases. This results in extended hospitalization, immobility, and rehabilitation for patients.
Patient-specific implants
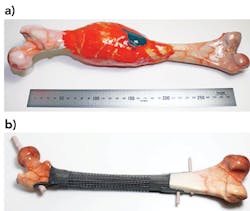
Patient-specific implants (PSIs), as opposed to traditional orthopedic implants, are derived from a patient’s own CT and MRI scans. The design process typically involves use of image processing software to segment a patient’s anatomy, identify the defect, disease, or deformity, prepare a virtual surgical plan, and design an implant. The implants are then manufactured by using additive manufacturing methods, traditional machining methods, or a combination of both. PSIs provide treatment options to patients who do not qualify for treatment with standard devices or are at a risk of amputation and ensuing severe disability. Use of PSIs has increased in recent years due to access to high-fidelity medical imaging modalities, image processing, and design software tools and additive manufacturing.
However, there exist multiple challenges that hinder uptake of PSIs. One such major challenge is extended lead time, which varies between 4 to 8 weeks depending on case complexities, number of design iterations, and manufacturing processes used. PSIs are mostly designed manually using conventional computer-aided drafting (CAD) tools or mesh manipulation tools. As a result, the design process is slow and prone to user-dependent variations. Sluggish uptake of PSIs is also due to lack of clinical data as a result of low volumes and uniqueness of each one-off ‘compassionate use’ cases.
RMIT University researchers are addressing these challenges associated with traditional orthopedic implants and current PSIs with novel ‘just-in-time’ (JIT) design methodology and its validation through extensive experimental studies to create evidence. JIT methods make use of rapid and automated generation of lattice structures to structurally and anatomically reconstruct bone defects.
Lattice structures as orthopedic implants
Orthopedic implants are used to replace diseased or damaged bone segments and joints. Every implant surgery involves removing damaged tissue and replacing it with an artificial implant for structural stabilization. The damage is usually from arthritis, bone tumor, stress fractures, and bone deformities. To ensure longer survival rates, implant designs should adapt to the pathology, geometry, and structure of the surrounding tissue.
The bone typically undergoes complex loading consisting of tensile compressive bending as well as shear and torsional forces, and the implant is required to mimic this behavior. Additionally, the stiffness of the implant must be comparable to the bone’s stiffness in order to avoid physical damage and stress shielding to avoid bone resorption and implant failure. On the other hand, the yield strength of the implant material should exceed that of the local bone so that the implant material can transfer loads and ground reactions to surrounding tissue without permanent deformation. Furthermore, the implant should possess superior fatigue resistance to sustain intermittent and high cyclic loading.
Lattice structures offer this ability to tailor mechanical properties and behavior, which lend themselves perfectly to orthopedic implant design. Lattice structures have traditionally been used in orthopedic implants as porous surface coatings on solid implants to enhance secondary fixation through bone ongrowth and ingrowth. In addition to their superior biological properties, lattice structures also possess unique structural properties, making them a superior alternative to solid implants. For example, lattice structures have high strength-to-weight compared to solid structures and their design variables can be tailored to enhance biological response as well as static and dynamic mechanical behavior, as demonstrated by RMIT University research studies.
JIT algorithms developed by RMIT University researchers rapidly design patient-specific orthopedic implants comprising smart lattice structures that are conformal to patient’s anatomy and iteratively optimized to the patient-specific biomechanical loads. The algorithms make use of extensive research on geometry-specific process parameters and associated manufacturing capability maps of additive manufacturing technology to ensure manufacture of error-free and high-fidelity lattice structures. The lattice implants designed using RMIT University methodology6 have several unique and novel features, including:
- The lattice implants are designed based on patient-specific image data and conform to the patient’s bone geometry.
- Automated algorithms generate the lattice structures rapidly to fill bone defects while ensuring complete manufacturability without the need for internal support structures.
- Lattice configuration consists of functionally varying pore sizes and shapes to simultaneously enhance bone-in growth and strength-to-weight ratio.
- The lattice structures are topologically optimized to withstand patient-specific loading conditions.
- The lattice configurations are tailored to match stiffness of the surrounding bone while exceeding the static and dynamic strength requirements.
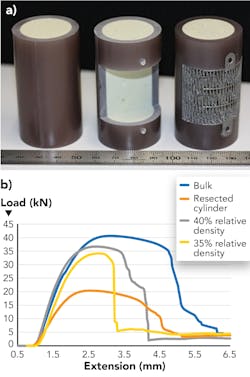
The algorithms used in this methodology are computationally highly efficient and can yield optimized designs within minutes, making iterative design feasible with potential to reduce lead time significantly. RMIT University researchers have also shown that mechanical properties and behavior can be tailored to achieve desired performance from load-bearing lattice implants. Several implants were designed and mechanically tested against the healthy bone geometry using synthetic bones.
The results illustrated in FIGURE 4 show that the implants designed using RMIT University methodology not only recover the load-carrying capacity of the bone, but they also mimic the stiffness of the substrate bone, yielding a superior option to conventionally designed implants.Summary
SLM technology offers opportunities for the manufacture of a range of new products. Lattices are structures that lend themselves perfectly to SLM and show promise in particular for biomedical implants. The RMIT University Centre for Additive Manufacturing is involved in a number of research projects with collaborators at Stryker South Pacific, St. Vincent’s Hospital, the Peter MacCallum Cancer Centre, and UTS, and supported by the IMCRC and ARC Training Centre in Additive Biomanufacturing to research and develop next-generation biomedical products using SLM technology.
REFERENCES
1. See www.astm.org/committee/f42.htm.
2. M. Scheffler and P. Colombo, Cellular Ceramics: Structure, Manufacturing, Properties and Applications, Wiley (2006); ISBN: 978-3-527-31320-4.
3. M. McMillan, M. Jurg, M. Leary, and M. Brandt, Rapid Prototyp. J., 23, 3, 486-494 (2017); doi:10.1108/rpj-01-2016-0014.
4. M. F. Ashby, Philos. Trans. A Math Phys. Eng. Sci., 364, 1838, 15–30 (Jan. 15, 2006); doi:10.1098/rsta.2005.1678.
5. M. Mazur et al., Int. J. Adv. Manuf. Tech., 84, 5, 1391–1411 (2016); doi:10.1007/s00170-015-7655-4.
6. D. Shidid, M. Leary, P. Choong, and M. Brandt, Phys. Procedia, 83, 4–14 (2016); doi:10.1016/j.phpro.2016.08.002.
DARPAN SHIDID, MARTIN LEARY, and MILAN BRANDT ([email protected]) are with the Centre for Additive Manufacturing at RMIT University, Melbourne, Australia; www.rmit.edu.au.